News
Medical 3D Technologies: From Scanning to Printing
April 2024
When we think of 3D technologies, we often think of 3D printing, which, as discussed later, has seen rapid advances in recent years.
However, a sometimes overlooked area of medical innovation is 3D scanning. Innovation in medical 2D scanning has been around for a long time, possibly the most famous example being Marie Curie’s mobile X-ray vehicle in World War 1. Computed Tomography (CT) scanning and other 3D scanning techniques have also been around for a long time. In recent years, however, thanks to the availability of higher computational power and significant advances in imaging technology, 3D scanning in general has seen innovation pace rapidly increase.
The hypothesis explored in this article is that, with medical 3D printing innovation moving forward at an ever-increasing rate, it is intuitive that medical 3D scanning innovation will follow. This is due to the benefits available in creating medical devices or equipment suited to specific patient characteristics or needs, but this requires the ability to identify these characteristics or needs more precisely.
The rise of medical 3D printing
In September 2023, the European Patent Office (EPO) published a patent landscape report entitled “Innovation trends in additive manufacturing: Patents in 3D printing technologies.”1 In this report, the authors identified the number of independent patent families which were published between 2001 and 2020 in the field of additive manufacturing and found that the majority were in the health and medical fields. There were 9,665 international patent families published over nearly two decades, and the number began to rapidly increase in 2013, a trend confirmed by the first graph presented below.
The appeal of 3D printing for medical technology is clear to see. It offers the ability to generate bespoke and patient-specific designs, it allows the production of parts which have very complex geometries, it enables parts to be designed with mechanical or chemical characteristics which better integrate with living tissue, and it generally uses less material than parts made using subtractive manufacturing.
During its rise to prominence, 3D printing was seen by some as a prohibitively expensive solution. However, in a 2016 study by the National Institute of Standards and Technology (NIST), entitled “Costs and Cost Effectiveness of Additive Manufacturing: A Literature Review and Discussion”2 the authors determined that the average price of additive manufacturing decreased by 51% between 2001 and 2011.
Therefore, with higher demands in the medical technology sector, rapid advancement in 3D printing capabilities, and dramatically reducing costs, it is unsurprising that 3D printing has been revolutionising the medical technology industry over the last decade.
The patent numbers
One way to look at trends in technological innovation is to look at patent filing numbers. Of course, not every innovator files a patent application, but, broadly speaking, patent filing numbers follow similar trends to innovation in the same technological area.
A broad assessment of these trends can be obtained by searching for the number of patent families with certain technological classification codes.
The graph below shows two patent trends between 2005 and 2023, namely:
- the number of patent families related to medical 3D scanning.
- the number of patent families related to medical 3D printing.
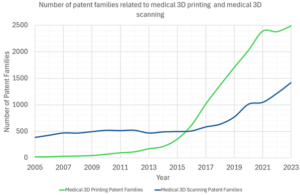
As can be seen in this graph, medical 3D printing family numbers began to increase rapidly in 2013, which is consistent with the EPO report. Medical 3D scanning family numbers began to increase at a greater rate after 2015.
Correlation between 3D printing and 3D scanning
Whilst the innovation in 3D printing technology and in 3D scanning technology is most likely to have happened independently, it is interesting to look for correlation across the two technology areas.
A starting point is to look at the total number of families related to both medical 3D printing and medical 3D scanning. These results are shown in the following graph.
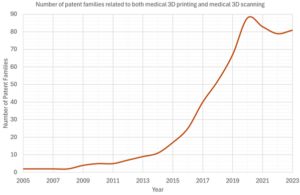
The rapid increase from 2013 is expected because the rapid increase in medical 3D printing families was likely to include some classifications relating to 3D scanning.
More interesting, though, is when the number of patent families relating to both medical 3D printing and medical 3D scanning is presented as a percentage of the total number of patent families related to medical 3D printing. This is shown in the following graph, starting in 2015 when all three of the previous trends were experiencing rapid increases.
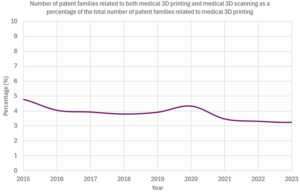
As can be seen, since 2015 this has consistently been between 3% and 5%. In other words, between 3% and 5% of medical 3D printing patent families are also classified as relating to 3D scanning.
Conclusion
Whilst it is unlikely that medical 3D printing innovation leads directly to 3D scanning innovation, and vice versa, these numbers show that medical 3D scanning innovation is increasing in pace at a similar rate to medical 3D printing innovation.
This is good news.
As medical 3D printing becomes more innovative this means that medical devices and equipment can become more and more suited to specific patient needs. However, a limitation could be reached whereby these needs cannot be identified accurately or efficiently.
Therefore, advancement in medical 3D scanning technology might contribute to ensuring that this limitation is not reached.
References
1Innovation trends in additive manufacturing Patents in 3D printing technologies. (2023). Available at: https://link.epo.org/web/service-support/publications/en-additive-manufacturing-study-2023-full-study.pdf.
2Thomas, D.S. and Gilbert, S.W. (2014). Costs and Cost Effectiveness of Additive Manufacturing. Costs and Cost Effectiveness of Additive Manufacturing. Available at https://nvlpubs.nist.gov/nistpubs/specialpublications/nist.sp.1176.pdf
































